|
Rassegna Stampa Scientifica Agosto 2022
|
State of the Art Review
Impact of vaping on respiratory health
BMJ 2022;378:e065997 (Published 18 July 2022)
Andrea Jonas
https://www.bmj.com/content/378/bmj-2021-065997
Breaking Nicotine’s Powerful Draw
Millions of smokers could be forced to confront the agony of nicotine withdrawal as the F.D.A. weighs calling for a drastic reduction in the addictive lure of cigarettes.
Aug. 2, 2022
At some point in the next few years, the 30 million smokers in the United States could wake up one day to find that cigarettes sold at gas stations, convenience stores and smoke shops contain such minuscule amounts of nicotine that they cannot get their usual fix when lighting up.
Would the smokers be plunged into the agonizing throes of nicotine withdrawal and seek out their favorite, full-nicotine brand on illicit markets, or would they turn to vaping, nicotine gum and other less harmful ways to get that angst-soothing rush?
Such scenarios inched closer to the realm of possibility in June, when the Food and Drug Administration said that it would move toward slashing nicotine levels in cigarettes in an effort to reduce the health effects of an addiction that claims 480,000 lives a year.
The agency set next May as its timetable for introducing a fully developed proposal. But many experts hope regulators will champion an immediate 95 percent reduction in nicotine levels — the amount federally funded studies have determined is most effective for helping smokers kick the habit.
It could be years before any new policy takes effect, if it survives opposition from the tobacco industry. Even so, health experts say any effort to decrease nicotine in cigarettes to nonaddictive levels would be a radical experiment, one that has never been implemented by any other country.
The science of nicotine addiction has come a long way since 1964, when a U.S. Surgeon General report first linked smoking to cancer and heart disease, although it would take another two decades for the mechanics of nicotine dependence to be understood and widely accepted.
Tobacco contains more than 7,000 chemicals, many of them harmful when burned and inhaled, but it is nicotine that keeps smokers coming back for more. Nicotine stimulates a surge of adrenaline in the brain while indirectly producing a flood of dopamine, the chemical that promotes feelings of contentment and relaxation. The effects, however, are short-lived, which is why heavy smokers need a fresh injection a dozen or more times a day.
Eric Donny, a tobacco expert at Wake Forest University School of Medicine who has conducted experiments with low nicotine cigarettes, says many scientists have come to embrace a 95 percent reduction in nicotine levels as ideal for helping study subjects smoke less. Anything higher, he said, can encourage participants to engage in so-called compensatory smoking — inhaling more deeply or smoking more frequently.
The studies he and other scientists have run recently used genetically modified tobacco bred to express less nicotine; bringing nicotine down to zero is not an option under the Tobacco Control Act, a 2009 law that gave the F.D.A. the power to regulate the manufacture and marketing of tobacco.
“When you get the nicotine in tobacco low enough, you just can’t get enough nicotine to maintain the dependence,” Dr. Donny said. “Smoking more creates adverse effects, like nausea, because the lungs can only handle so much of a burned substance.”
But even as tobacco control researchers cheered the F.D.A. announcement, they acknowledged that any move to lower nicotine in cigarettes would be enormously challenging for inveterate smokers — even among the 70 percent who have said they would like to stop. As it is, fewer than one in 10 adults who try to quit smoking succeed, a reflection of nicotine’s addictive prowess and the limitations of nicotine replacement therapy.
Dr. Nora Volkow, director of the National Institute on Drug Abuse, expressed confidence in the studies that backed an immediate cut in nicotine levels versus a gradual tapering. But she said that scientists and regulators still needed to address the welter of unforeseen consequences that could prove disruptive to determined smokers and could fuel the creation of underground markets for full-nicotine cigarettes. “You cannot completely predict outcomes based on a clinical randomized study,” she said. “Biology and life are not so precise.”
Some scientists have urged caution for any plan that would drastically cut nicotine levels in one fell swoop, warning that the existing research on low-nicotine cigarettes is imperfect, given the high number of study participants who cheat. The skeptics, among them tobacco company executives, warn that banning conventional cigarettes would drive determined smokers to seek imports from Mexico and Canada. They also argue that some smokers, including teenagers, could develop a habit that pairs vaping or nicotine gum with low-nicotine cigarettes, which are just as carcinogenic as traditional cigarettes.
Lynn T. Kozlowski, a tobacco researcher at the University at Buffalo who has contributed to four Surgeon General reports on smoking since 1981, said nicotine was a highly addictive drug, with a stranglehold on users that could rival cocaine and heroin, and that the F.D.A. needed to consider how a sweeping decrease of nicotine in cigarettes would affect smoker behavior.
“What scares me is a national experiment with very low nicotine cigarette that is done without some testing in the real world,” he said. The studies many experts cite when promoting a 95 percent drop in nicotine levels relied upon paid participants, he noted, adding that some of them secretly smoked their own brands at the same time that researchers were plying them with low-nicotine cigarettes.
In interviews, smokers who had heard about the F.D.A.’s announcement said they were conflicted by the prospect of being forced to abandon their addiction, despite knowing full well that it damaged their health and would likely shorten their life span. Mike Harrigan, an options trader who was taking a smoking break outside the Chicago Board of Trade, said he feared he might actually end up smoking more if cigarettes contained significantly lower amounts of nicotine. “It may help newer smokers, but it will hurt people who are used to a certain level of nicotine,” said Mr. Harrigan, 55, who has been a pack-a-day smoker for three decades.
Dr. Kozlowski said he was especially concerned by the agency’s mixed messaging and seemingly conflicted stance on e-cigarettes, which deliver nicotine without the tar and many other toxins that are inhaled when tobacco is ignited. Even if the long-term impacts of vaping remain unknown — though health experts agree that teenagers should be discouraged from trying e-cigarettes — there is mounting consensus that such products are useful for helping adult smokers quit.
The F.D.A. has so far approved just six vaping products and has denied more than a million others, including those made by Juul Labs. Earlier this summer, the agency ordered Juul off shelves, citing the potential harm from chemicals that could leach out of its e-liquid cartridges. But the F.D.A. has since granted the company further review.
Dr. Judith Prochaska, an addiction specialist at Stanford University who runs a smoking cessation clinic for patients with cancer and their families, said lighting up during a stressful phone call, while sipping a cocktail or following a meal creates a powerful memory that conditions the mind into associating a cigarette with the stimulation or succor that it delivers via the rush of nicotine.
“All these everyday behaviors cue your brain that nicotine is coming,” she said. “It’s basically the Pavlovian dog effect but conditioned here with a highly addictive drug.”
Over time, the dependency deepens. Regular smoking promotes the formation of additional dopamine receptors — sometimes millions more. When a smoker goes cold turkey, those unrequited receptors prompt the anxiety, irritability and depression that can make nicotine withdrawal so hard to bear.
Nicotine patches, gum and vapes can help to satisfy some of the cravings, but they cannot replace the rituals of having a cigarette: the retreat outside with a co-conspirator, the crinkling of cellophane and foil as you open a new pack, the heady buzz of that first drag.
Bruce Holaday, 69, a retired educator from Mill Valley, Calif., knows full well the power of nicotine. Over the past five decades, Mr. Holaday reckons he has tried to quit 100 times, often relying on nicotine replacement products. But he invariably returned to his lifelong, pack-a-day affair with Marlboro Lights.
His last attempt in August, a cold turkey gambit without nicotine replacement therapy, triggered an excruciating maelstrom of cravings that lasted several months. “It was like a sudden earthquake of desire and need, and then there would be these tremors for the next 10 to 15 minutes,” he said.
But this time, Mr. Holaday joined a support group at Stanford Health Care, which introduced a powerful social component into his quest. He described the effect as “not wanting to let the team down” and said he learned to avoid stressful situations, like watching the news. He discovered that if he could face down the initial waves of craving, they invariably subsided.
In late June, he passed the one-year mark since taking his last drag.
He gained weight but no longer gets easily winded on hikes. And he is confident he will never go back to smoking.
Asked about the prospect of drastic government intervention to compel Americans to quit, Mr. Holaday paused and thought about the first puff he took a half-century ago as a college freshman. “Without that nicotine rush, I would have probably walked away and never smoked again,” he said. “It will be rough for smokers, but anything we can do to prevent a new generation from getting hooked is a good thing.”
Robert Chiarito contributed reporting from Chicago.
Andrew Jacobs is a health and science reporter, based in New York. He previously reported from Beijing and Brazil and had stints as a metro reporter, Styles writer and national correspondent, covering the American South. @AndrewJacobsNYT
A version of this article appears in print on Aug. 2, 2022, Section D, Page 1 of the New York edition with the headline: A Plan to Ease the Cravings of Smokers.
https://www.nytimes.com/2022/08/02/health/fda-nicotine-addiction.html
Person-years of life lost and lost earnings from cigarette smoking-attributable cancer deaths, United States, 2019
International Journal of Cancer
First published: 10 August 2022
Farhad Islami, Emily C. Marlow, Jingxuan Zhao, Daniel Wiese, Samuel Asare, Priti Bandi, Blake Thomson, Zhiyuan Zheng, Nigar Nargis, K. Robin Yabroff, Ahmedin Jemal
https://onlinelibrary.wiley.com/doi/full/10.1002/ijc.34217
Related coverage:
Smoking Still Ends 123,000 American Lives Each Year
Note: The information in the article accurately reflects the study findings, but the headline omits the reference to cancer deaths. The overall toll of tobacco-related mortality in the US is generally cited as 480,000 annual deaths.
“In this national cohort study of 17 073 children with neuroimaging outcomes, a significant association was found of early-age initiation of tobacco use with lower crystalized cognition composite score and impaired brain development in total cortical area and volume. Region of interest analysis also revealed smaller cortical area and volume across frontal, parietal, and temporal lobes… These findings suggest that youths vulnerable to e-cigarettes and tobacco products should be treated as a priority population in tobacco prevention.”
Longitudinal Assessments of Neurocognitive Performance and Brain Structure Associated With Initiation of Tobacco Use in Children, 2016 to 2021
JAMA Netw Open. 2022;5(8):e2225991.
August 10, 2022
Hongying Daisy Dai, Gaelle E. Doucet, Yingying Wang, Troy Puga, Kaeli Samson, Peng Xiao, Ali S. Khan
https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2794988
Related JAMA Netw Open Commentary:
Understanding the Association of Childhood Tobacco Use With Neuropathological Outcomes and Cognitive Performance Deficits in Vulnerable Brains
https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2794992
Note: Open Access.
“The FCTC [Framework Convention on Tobacco Control] offers a framework to interpret state obligations to progressively realize the human right to health, with Article 14 and its guidelines providing a basis to clarify state obligations to ensure that those addicted to tobacco receive cessation support. However, Article 14 implementation has been limited. The right to health provides an alternative basis under international law to recognize the inherent dignity of those addicted to tobacco, implement FCTC
obligations under Article 14 and facilitate state accountability.”
Could international human rights obligations motivate countries to implement tobacco cessation support?
Addiction
First published: 06 July 2022
Benjamin Mason Meier, Martin Raw, Donna Shelley, Chris Bostic, Anahita Gupta, Kelsey Romeo-Stuppy, Laurent Huber
https://onlinelibrary.wiley.com/doi/abs/10.1111/add.15990
Person-years of life lost and lost earnings from cigarette smoking-attributable cancer deaths, United States, 2019
International Journal of Cancer
First published: 10 August 2022
Farhad Islami, Emily C. Marlow, Jingxuan Zhao, Daniel Wiese, Samuel Asare, Priti Bandi, Blake Thomson, Zhiyuan Zheng, Nigar Nargis, K. Robin Yabroff, Ahmedin Jemal
https://onlinelibrary.wiley.com/doi/full/10.1002/ijc.34217
Related coverage:
Smoking Still Ends 123,000 American Lives Each Year
Note: The information in the article accurately reflects the study findings, but the headline omits the reference to cancer deaths. The overall toll of tobacco-related mortality in the US is generally cited as 480,000 annual deaths.
“In this national cohort study of 17 073 children with neuroimaging outcomes, a significant association was found of early-age initiation of tobacco use with lower crystalized cognition composite score and impaired brain development in total cortical area and volume. Region of interest analysis also revealed smaller cortical area and volume across frontal, parietal, and temporal lobes… These findings suggest that youths vulnerable to e-cigarettes and tobacco products should be treated as a priority population in tobacco prevention.”
Longitudinal Assessments of Neurocognitive Performance and Brain Structure Associated With Initiation of Tobacco Use in Children, 2016 to 2021
JAMA Netw Open. 2022;5(8):e2225991.
August 10, 2022
Hongying Daisy Dai, Gaelle E. Doucet, Yingying Wang, Troy Puga, Kaeli Samson, Peng Xiao, Ali S. Khan
https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2794988
Related JAMA Netw Open Commentary:
Understanding the Association of Childhood Tobacco Use With Neuropathological Outcomes and Cognitive Performance Deficits in Vulnerable Brains
https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2794992
Note: Open Access.
“The FCTC [Framework Convention on Tobacco Control] offers a framework to interpret state obligations to progressively realize the human right to health, with Article 14 and its guidelines providing a basis to clarify state obligations to ensure that those addicted to tobacco receive cessation support. However, Article 14 implementation has been limited. The right to health provides an alternative basis under international law to recognize the inherent dignity of those addicted to tobacco, implement FCTC
obligations under Article 14 and facilitate state accountability.”
Could international human rights obligations motivate countries to implement tobacco cessation support?
Addiction
First published: 06 July 2022
Benjamin Mason Meier, Martin Raw, Donna Shelley, Chris Bostic, Anahita Gupta, Kelsey Romeo-Stuppy, Laurent Huber
https://onlinelibrary.wiley.com/doi/abs/10.1111/add.15990

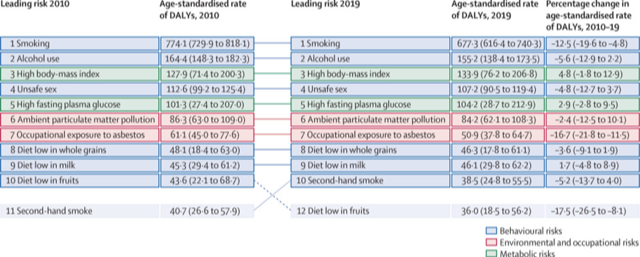
“Globally, there has been substantial progress in reducing exposure to tobacco that can be linked to coordinated international and national prevention efforts. Interventions through taxation and regulatory policies for tobacco smoking, including smoke-free policies, increased tobacco taxes, and advertisement bans guided by the WHO Framework Convention on Tobacco Control, have played a major role in these efforts… Smoking continues to be the leading cancer risk factor globally, with other substantial contributors to cancer burden varying around the world.”
The global burden of cancer attributable to risk factors, 2010–19: a systematic analysis for the Global Burden of Disease Study 2019
The Lancet
VOLUME 400, ISSUE 10352, P563-591, AUGUST 20, 2022
Published: August 20, 2022
GBD 2019 Cancer Risk Factors Collaborators
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(22)01438-6/fulltext
https://www.thelancet.com/action/showPdf?pii=S0140-6736%2822%2901438-6
Related coverage:
Smoking, alcohol, high BMI leading causes of global cancer deaths: Lancet study
Smoking, Alcohol, High BMI Main Causes Of Global Cancer Deaths: Lancet
“Findings pertaining to the effects of tobacco product use on the incidence of SARS-CoV-2 infection are inconsistent. However, evidence supports a role for cigarette smoking in increasing the risk of poor COVID-19 outcomes, including hospital admission, progression in disease severity, and COVID-19-related mortality… A deeper understanding of the links between tobacco product use and disease risk could help to shape public health recommendations and to improve the clinical care of those with a history of tobacco dependence.”
PERSONAL VIEW
Tobacco product use and the risks of SARS-CoV-2 infection and COVID-19: current understanding and recommendations for future research
Lancet Resp Med
Published: August 16, 2022
Neal L Benowitz, Maciej L Goniewicz, Bonnie Halpern-Felsher, Suchitra Krishnan-Sarin, Pamela M Ling, Richard J O'Connor, Mary Ann Pentz, Rose Marie Robertson, Aruni Bhatnagar
https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(22)00182-5/fulltext
|
Rassegna Stampa Scientifica Settembre 2022
|
Juul Settles Multi-State Youth Vaping Inquiry for $438.5 Million
The tentative deal would close a yearslong investigation by nearly three dozen states into the company’s marketing and sales practices.
Juul Labs has tentatively agreed to pay $438.5 million to settle an investigation by nearly three dozen states that focused on the company’s sales and marketing practices that they claim fueled the teenage vaping crisis.
The investigation found that the company appealed to young people by hiring young models, using social media to court teenagers and giving out free samples. William Tong, Connecticut’s attorney general, said in a news conference that the investigation found that the company had a “porous” age verification system for its products and 45 percent of its Twitter followers were ages 13 to 17.
“We think that this will go a long way in stemming the flow of youth vaping,” Mr. Tong said. “We are under no illusions and cannot claim that it will stop youth vaping. It continues to be an epidemic. It continues to be a huge problem. But we have essentially taken a big chunk out of what was once a market leader.”
Juul said on Tuesday that the settlement “is a significant part of our ongoing commitment to resolve issues from the past. The terms of the agreement are aligned with our current business practices which we started to implement after our companywide reset in the fall of 2019.”
But the company said it was not acknowledging any wrongdoing in the settlement.
The tentative settlement prohibits the company from marketing to youth, from funding education in schools and misrepresenting the level of nicotine in its products. Juul had already discontinued several marketing practices and withdrawn many of its flavored pods that appealed to teenagers, under public pressure from lawmakers, parents and health experts a few years ago when the vaping crisis was at a peak.
The company’s ability to continue to sell its products has been in question in recent months. The Food and Drug Administration announced in June that it was denying the company’s request for marketing authorization to sell the vapes, saying its application lacked evidence to prove they would benefit public health. The agency also cited “insufficient and conflicting” data from the company. The company quickly went to court and got a temporary reprieve.
The company responded days later that it helped two million adult smokers quit combustible cigarettes and took exception with the agency’s conclusions about chemicals in its products. The F.D.A. then announced that it would allow the products to stay on the market pending additional review of “scientific issues.”
Christina Jewett covers the Food and Drug Administration. She is an award-winning investigative journalist and has a strong interest in how the work of the F.D.A. affects the people who use regulated products. @By_Cjewett
https://www.nytimes.com/2022/09/06/health/juul-settlement-vaping-crisis.html
"Nicotine gummies are a public health crisis just waiting to happen among our nation's youth, particularly as we head into a new school year," FDA [Food & Drug Administration] Commissioner Dr. Robert Califf warned last month. The FDA last month sent a warning letter to VPR Brands, one of the companies that makes nicotine gummies, advising that the products were being sold illegally. By law, manufacturers need to submit an application and have it approved by the FDA before a tobacco product can be legally marketed in the United States. The agency says VPR had not asked for this "premarket authorization" for the gummies. Each of the gummies had 1 milligram of nicotine, and they came 12 to a package. The FDA says 1 to 4 milligrams of nicotine could be severely toxic to children under 6 as well as to older children, depending on their weight.” [Jen Christensen. Nicotine gummies are a 'public health crisis just waiting to happen,' FDA says, CNN]
“Non-tobacco oral nicotine products were the second most prevalent nicotine product used by adolescents. They were disproportionately used by certain racial or ethnic, sexual, or gender minority groups, and those with a history of nicotine use. Adolescent non-tobacco oral nicotine product use surveillance should be a public health priority.”
Adolescent Use of Flavored Non-Tobacco Oral Nicotine Products
Pediatrics
Volume 150, Issue 3, September 2022
Online First: August 08 2022
Alyssa F. Harlow, Erin A. Vogel, Alayna P. Tackett, Junhan Cho, Dae-Hee Han,Melissa Wong, Myles G. Cockburn, Steve Y. Sussman, Jennifer B. Unger, Adam M. Leventhal, Jessica L. Barrington-Trimis
Note: Open Access.
US: Kennedy Center Promotes Big Tobacco; NZ: Convenience Store Lobby
“With all we know now about the tobacco industry’s tactics, discriminatory marketing, targeting of youths and addictive, deadly products, what would he think about the institution bearing his name promoting cigarette companies? The Met and others brought down the curtain on their Sackler [opioid-pushing Purdue Pharma] sponsorships. If it’s serious about its social impact, it’s time the Kennedy Center ended its dance with Big Tobacco.” [The Kennedy Center should stop promoting Big Tobacco, Washington Post]
“Let us carry on peddling death, or our businesses will die, dairy owners have told MPs, though not in those exact words… The Smokefree Environments and Regulated Products (Smoked Tobacco) Amendment Bill is designed to “significantly” limit the number of retailers allowed to sell smoked tobacco products. Fewer places selling “cancer sticks” will mean fewer opportunities for young people to start smoking, and will mean it’s easier for smokers to quit, supporters argue… But if dairies (sic) primary function is peddling cigarettes, the closure of dairies sounds like a gain for the community. And given the choice between dead people, and dead businesses, the people win.” [Rob Stock. How can dairies survive if their business is built on smokes?, Stuff (NZ)]
“Shocking new footage suggests you might want to make sure you're using the correct charger for your e-cigarette. Video from experts at London charity Electrical Safety First shows a powerful explosion from a small lithium ion battery inside an e-cigarette… [J]ust like smartphones and electric cars, e-cigarettes contain lithium-ion batteries that can burst into flames or explode if pierced, damaged or overheated… Brits may already be reconsidering keeping their vape in their pocket after instances of the devices exploding and causing horrific burns.” [Jonathan Chadwick. Do you vape? Check your charger NOW: Shocking video shows the lithium battery from an e-cigarette EXPLODING during charging test, Daily Mail]
Opinion
The Kennedy Center should stop promoting Big Tobacco
By Rebecca Perl
September 9, 2022

A view from the Roosevelt Bridge of the Reach, a new multiuse complex at the Kennedy Center in D.C. (Bill O'Leary/The Washington Post)
Rebecca Perl, a former health and science reporter for The Post, is vice president of partnerships and initiatives for Vital Strategies, a partner in tobacco industry watchdog, STOP and advises governments and nongovernmental organizations on communication strategies for tobacco control campaigns around the world as part of the Bloomberg Initiative to Reduce Tobacco Use.
On Sept. 17, the John F. Kennedy Center for the Performing Arts in D.C. will launch a new exhibit dedicated to its namesake. Advance media coverage praises the center’s investment in its social impact, in line with Kennedy’s ethos and values. But as is often the case when organizations talk about being good citizens, there are gaps between the rhetoric and the reality.
For the Kennedy Center, it is taking money from some of the world’s largest tobacco corporations. Instead of rejecting hundreds of thousands of dollars gained through the sale of products linked to addiction, preventable disease and premature death, the center recently added global cigarette giant Philip Morris International (PMI) as a new corporate sponsor, alongside its long-standing relationship with PMI’s former parent company, Marlboro-maker Altria Group. Combined, they are contributing at least half a million dollars annually — a testimony to a never-ending dance of the underfunded arts with deep-pocketed corporations looking to burnish their reputations.
Yet, despite funding challenges, especially post-coronavirus, other world-class venues are making an ethical choice to end sponsorships from problematic donors. For example, when the Sackler family’s role in the opioid crisis was exposed, the Metropolitan Museum of Art in New York ended its 50-year relationship. The Louvre in Paris and the Guggenheim in New York and many others also dropped the Sackler name. The opioid epidemic claims more than 68,000 lives a year in the United States. The tobacco epidemic? More than 480,000.
The Kennedy Center’s commitments to better serving youths and people of color ring especially hollow while it’s helping the tobacco industry reach groups who have been targeted for decades with deceptive and even racially targeted marketing. Menthol cigarettes, promoted heavily in Black media and in predominantly Black neighborhoods, have caused disproportionate harm to Black people. Products such as nicotine pouches and e-cigarettes are believed to be hooking a new generation.
Visiting the Kennedy Center to see “The Nutcracker” last winter, I could see the Altria-sponsored 50th-anniversary theater season was packed with family- and teen-friendly productions. Attaching Altria’s name to productions for young people reminds me of Chinese tobacco companies sponsoring schools in China and other countries.
What will it take to break the Kennedy Center’s addiction to tobacco dollars? The revelation that industry executives stood before Congress a few miles away, claiming that nicotine wasn’t addictive when the companies knew that wasn’t true, wasn’t enough.
The Kennedy Center didn’t drop Altria when it invested heavily in Juul, after the Food and Drug Administration named the company as a leading player in the United States’ youth vaping epidemic. Nor when Altria spoke out against the FDA’s proposed menthol ban, which could help save up to 6,000 Black lives each year.
Instead, Altria’s brands remain etched into the center’s marble walls, including at the newest Kennedy Center venue, the Reach, which aims to embody “President Kennedy’s vision” and reach a diverse audience through education, youth programs and community outreach. Altria and PMI are on the Kennedy Center’s website, and Altria is splashed across its 50th-anniversary materials, in social media posts, newsletters and performance programs, even celebrated as “a donor that makes a difference” in the 50th-anniversary magazine. Altria’s chief executive is a vice chair of the Kennedy Center’s Corporate Fund Board, alongside PMI’s president of the Americas.
It’s easy to see why the Kennedy Center’s youth-friendly 50th season and the Reach’s youth and community audience appeal to Altria and PMI. As more smokers die or quit, the companies need to tap into the next generation. Altria’s Marlboro and Juul brands are already popular with young Americans. Both companies might also hope to influence D.C. stakeholders in their favor — and that’s potentially bad news for health.
There is, however, something especially insidious about aligning the tobacco industry with performances aimed at preschoolers, children and other youths. Perhaps it is because such shows capture the dreams of little girls and boys and the ambitions of so many young people, who continue to be targeted by cigarette companies.
President Kennedy believed the arts should be a positive force in American life. With all we know now about the tobacco industry’s tactics, discriminatory marketing, targeting of youths and addictive, deadly products, what would he think about the institution bearing his name promoting cigarette companies? The Met and others brought down the curtain on their Sackler sponsorships. If it’s serious about its social impact, it’s time the Kennedy Center ended its dance with Big Tobacco.
https://www.washingtonpost.com/opinions/2022/09/09/kennedy-center-should-stop-promoting-big-tobacco/
How McKinsey Got Into the Business of Addiction
The consulting firm’s work with opioid makers is well known, but for decades McKinsey worked with Big Tobacco and has also advised Juul, the e-cigarette company.
McKinsey & Company in New York City. The firm’s work with opioid makers is well known, but its work with cigarette and vaping companies has escaped public scrutiny.Credit...John Taggart for The New York Times
By Walt Bogdanich and Michael Forsythe
Bogdanich and Forsythe, investigative reporters at The Times, are the authors of the forthcoming book “When McKinsey Comes to Town: The Hidden Influence of the World’s Most Powerful Consulting Firm,” from which this article is adapted.
Sept. 29, 2022
When McKinsey & Company, the global consulting giant, sat down with executives of Juul Labs in late 2017, the vaping company was well on its way to becoming a sensation among teenagers eager to latch on to the latest fad — inhaling flavored, supercharged nicotine vapor through a sleek new device easily hidden from parents and teachers.
With grand ambitions, Juul needed marketing advice from McKinsey, the most respected voice in consulting, to help it on its way to a valuation greater than the Ford Motor Company. For less than two years of work, McKinsey billed Juul $15 million to $17 million.
But the client came with a reputational risk, and McKinsey preferred to keep the arrangement secret. Although its product was conceived as a way to help adults stop smoking, Juul stood accused of marketing nicotine to teenage nonsmokers, addicting a new generation in much the same way the cigarette industry hooked their parents. This month, several years after McKinsey took the company as a client, Juul agreed to pay $438.5 million to settle government investigations into its marketing practices, though it did not acknowledge wrongdoing in the settlement. Those marketing practices had included using young models, social media and flavored nicotine.
McKinsey, which was not involved in the settlement, said its work with Juul had focused on youth vaping prevention. That work was just the latest in a decades-long history of consulting for companies that sell addictive products. The full story of McKinsey’s role in advising these companies — while also consulting for their government regulators — has never been told.
Last year, McKinsey agreed to pay more than $600 million to settle state investigations into its role in helping Purdue Pharma and other drugmakers fuel the opioid epidemic. And for decades, McKinsey has helped manufacturers boost sales of the most lethal consumer product in American history — cigarettes.
As recently as 2016, more than 50 years after the surgeon general confirmed the link between smoking and cancer, McKinsey still saw merit — and profits — in continuing to help companies sell more cigarettes.
In a slide deck prepared for Altria, formerly Philip Morris, McKinsey offered ideas for how the tobacco company could keep customers and lure new smokers. It presented a mock-up of what a Marlboro smartphone app would look like, complete with a way for loyal smokers to win points redeemable for small prizes.
McKinsey felt comfortable proposing ways for the manufacturer of Marlboro cigarettes to sell more of them.Credit...Dolly Faibyshev for The New York Times
McKinsey’s most important work for Juul Labs involved responding to an F.D.A. crackdown on youth vaping.Credit...Caroline Tompkins for The New York Times
“We are one team, working side-by-side,” McKinsey wrote in a slide deck prepared for Altria, illustrated with photos of cigarettes. McKinsey also advised Altria on marketing e-cigarettes, with the goal of making one of its products the “Nespresso of e-vapor” and stopped advising tobacco companies only last year.
McKinsey’s services are highly valued; its clients include many of the most respected blue-chip companies as well as governments around the world. For companies selling addictive products it also offered deep ties to the Food and Drug Administration, a regulatory agency vital to their survival. In four years under President Donald J. Trump, McKinsey took in $77 million in consulting contracts with the F.D.A.
McKinsey’s vaunted value system points to why a company so widely admired could end up working so long for tobacco companies. On one hand, McKinsey used those values to recruit the best and brightest students by suggesting that a job there means more than a big paycheck — it also offers the possibility of doing good, of helping those most in need. Yet McKinsey’s overriding value, No. 1 on its list — to put client interests first — created an environment in which client service sometimes trumped its own moral code.
McKinsey denies any wrongdoing in helping to market opioids, vaping and cigarettes — its trifecta of addiction clients — or that its F.D.A. contracts posed a conflict of interest, because it never advised the agency on any specific drug, a McKinsey spokesman said in a written response.
McKinsey agreed last year to pay more than $600 million to settle state investigations into its role in the opioid epidemic.Credit...Carolyn Kaster/Associated Press
Seth Green, a former McKinsey consultant who worked at the firm for two years and is now a dean at the University of Chicago, questioned the wisdom of rigidly adhering to the client-first dictum. “If we don’t bring a moral purpose to these businesses, then the purpose inevitably becomes the client and whatever the client is trying to achieve,” Mr. Green said.
Juul and the Rise of Youth Vaping
- ‘Move Fast and Vape Things’: From a fledgling start-up to Silicon Valley juggernaut, a Times documentary traced the e-cigarette maker’s path to a public health villain.
- Hooking a Generation: Juul said its e-cigarette was meant to give adult smokers a safer alternative to cigarettes — not hook young people on nicotine. The reality inside the company was different.
- The Price of Cool: E-cigarettes may help tobacco smokers quit. But the alluring devices can swiftly induce a nicotine habit in teens who never smoked.
- Vaping Loophole: The Food and Drug Administration’s crackdown in 2020on flavored e-cigarettes like Juul was meant to curtail teen vaping. Some companies moved just beyond the agency’s reach by swapping out one key ingredient.
Even if that means consulting for Big Tobacco.
Teenagers’ Favorite Flavors
Alfonso Pulido, a McKinsey partner, arrived at the San Francisco offices of the Covington & Burling law firm on a mid-October morning last year to do something antithetical to the firm’s strict code of secrecy: talk about McKinsey’s work with clients, specifically Juul and Altria.
Mr. Pulido was there to give a deposition in connection with an unresolved product liability case filed in a federal court in California. In the deposition, marked highly confidential, Mr. Pulido said McKinsey first discussed doing business with Juul in 2015 when it proposed doing a risk assessment of Juul’s tentative plan to enter the marijuana market.
McKinsey chose not to do the study, Mr. Pulido said, because marijuana “was not regulated or legal at the time.”
It wasn’t until 2017 that the consultancy performed a pricing study for Juul’s vaping device. Afterward, McKinsey offered advice on branding, organization, retail, flavor evaluation, youth vaping prevention and regulatory issues. The company also consulted for Altria, which was trying to muscle into the vaping business.
Flavored nicotine had become highly controversial because health care experts blamed Juul for using flavors that appealed to young people.
Mr. Pulido acknowledged that McKinsey had surveyed teenagers as young as 13, asking them to rank flavor names in order of preference, though he emphasized that no sensory testing had taken place.
Esfand Nafisi, a lawyer representing clients who had sued Juul for marketing to children, pressed him on that answer.
“Did anyone at McKinsey stop and say, ‘Hey, maybe we shouldn’t be helping tobacco companies study teenagers’?”
“The stated objectives were to help inform youth prevention activity as well as responsibly introduce a flavor that was appealing to adult smokers,” Mr. Pulido responded.
“In retrospect, does McKinsey think this survey was appropriate?” Mr. Nafisi asked several minutes later.
“I don’t have an opinion on it,” Mr. Pulido responded. But he added that it “feels correct.”
The survey found that the favorite flavor name among ages 13 to 21 was mint, Mr. Pulido said.
“Did you know that mint would go on to become an incredibly popular flavor with teens a year after this Power Point deck was presented?” Mr. Nafisi asked.
“No, I was not aware of that.”
McKinsey records show that Bob Sternfels played an administrative role on the Juul team. McKinsey says he did not work on the account.Credit...Singapore Press, via Associated Press
The work with Juul attracted interest at the highest levels inside the firm. Bob Sternfels, now McKinsey’s managing partner, played an administrative role on the Juul account, an internal document shows. (A McKinsey spokesman said that while Mr. Sternfels knew a senior Juul executive, he had not worked on that account.)
McKinsey’s most important work for Juul involved responding to the F.D.A.’s crackdown on youth vaping. With the F.D.A. circling, demanding answers as to why teenagers were so attracted to Juul, the company asked McKinsey to help prepare a defense and respond to the agency’s inquiry.
The nature of that work remains a secret, because for those services McKinsey was paid through Juul’s law firm, Sidley Austin, allowing Mr. Pulido to claim lawyer-client privilege. “I know in some instances we are retained through legal counsel as part of privilege,” Mr. Pulido said.
At least one McKinsey partner, Michael Chui, grew concerned watching vaping spike in popularity, though it was not at all clear that he knew McKinsey had Juul as a client. (Consulting teams are not allowed to share information.)
“In just a few years, vaping has wiped out two decades of work getting teens to quit (or never start) cigarette smoking,” Mr. Chui wrote in a public comment on a magazine article about Juul.
Mr. Pulido said McKinsey stopped work with Juul in 2019 because of “increasing regulatory uncertainty and increased awareness of youth use.”
McKinsey and the Tobacco Industry
Vaping became popular as smoking rates across the nation began to decline in response to a drumbeat of scientific findings that cigarettes are highly addictive and deadly, facts that the companies knew but kept secret, choosing deception over disclosure.
McKinsey began counseling the tobacco industry in 1956, when researchers had already reported data suggesting that smoking appeared to cause cancer. Back then, Philip Morris hired McKinsey to conduct a wall-to-wall examination of its manufacturing operation. This was no cursory walk-through. Consultants visited plants, interviewed managers and studied sales figures.
In a subsequent study, McKinsey recommended how the company should set up its research department. The report was significant for another reason: It foreshadowed the industry’s transformation from selling a largely agricultural product to a scientifically engineered cigarette with fine-tuned nicotine levels. It cited the development of “reconstituted tobacco,” a manufacturing process that in subsequent years was shown to help achieve nicotine levels that researchers considered sufficient to ensure addiction.
Surgeon General Luther Terry, center, settled questions over the dangers of smoking when he announced to the nation that studies had confirmed the link between cigarettes and cancer.Credit...Getty Images
In 1964, Surgeon General Luther L. Terry settled questions over the dangers of smoking when he announced to the nation that studies had confirmed the link between cigarettes and cancer. Dr. Terry made his announcement on a weekend to minimize its impact on stock prices.
McKinsey now had another reason to back away from Big Tobacco. But the tobacco companies wanted to keep selling cigarettes, so McKinsey stayed to help them do just that. In addition to Philip Morris, the firm’s clients included R.J. Reynolds, Lorillard, Brown & Williamson, British American Tobacco and Japan Tobacco International.
More health warnings followed.
In 1992, the federal judge H. Lee Sarokin became so outraged reading internal industry documents produced in a liability lawsuit that he cast aside judicial restraint when he wrote: “Who are these persons who knowingly and secretly decide to put the buying public at risk solely for the purpose of making profits and who believe that illness and death of consumers is an appropriate cost of their own prosperity!”
In response to mounting criticism, in 1993 Lorillard’s chief executive, Andrew Tisch, asked employees to cooperate with McKinsey, assuring them that the consultants were “renowned for their ability to solve problems and create opportunity.”
By taking on Lorillard, McKinsey agreed to help a company whose best-selling cigarette by far was Newport, with its high nicotine content and menthol flavor. Menthol masked the harsh taste of burning tobacco, making it appealing for novice smokers. Nicotine took care of the rest, turning them into repeat customers.
McKinsey did allow employees to opt out of helping Big Tobacco, or any other industry they found objectionable, but finding replacements eager to impress senior partners critical to their advancement was usually easy.
In 2006, a federal judge, Gladys Kessler, delivered the harshest condemnation yet of cigarette makers, branding them civil racketeers, saying the industry had “marketed and sold their lethal product with zeal, with deception, with a single-minded focus on their financial success, and without regard for the human tragedy or social costs that success extracted.”
Ten years later, McKinsey still felt comfortable proposing ways for the manufacturer of Marlboro cigarettes to sell more of them.
‘Startled and Surprised’
Senator Christopher Dodd speaking in 2009 after Congress authorized the F.D.A. to regulate tobacco products. For advice, the agency subsequently went to McKinsey.Credit...Susan Walsh/Associated Press
After Congress gave the F.D.A. the authority to regulate tobacco products in 2009, the agency sought McKinsey’s wisdom on a variety of issues, though its leaders apparently were unaware that the firm had been guiding Big Tobacco’s development for decades. In subsequent years, the agency awarded the consultancy $11 million for advice on tobacco regulation and for organizing the F.D.A. office that includes tobacco regulation.
“We have served the F.D.A. on over 30 initiatives,” McKinsey wrote in securing $1.1 million to advise the agency’s Center for Tobacco Products about, among other things, “risk identification and mitigation” as well as “influencing the behaviors, opinions and practices that are contrary to the goals and objectives” of tobacco regulators. Presumably that included cigarettes.
Eric N. Lindblom, former director of the Office of Policy for the Center of Tobacco Products, said he was “startled and surprised” to learn of these apparent conflicts. “We didn’t think, duh, they are also going to serve the industry,” he said.
Dr. Lawrence Deyton, a physician and the tobacco center’s first director, said he had also been unaware of McKinsey’s work for cigarette companies. “That should have been disclosed,” he said. As late as 2019, McKinsey’s roster of tobacco clients included not just Altria but also Imperial Brands, British American Tobacco and Japan Tobacco International.
From 2018 through early 2020, McKinsey made at least $45 million in fees from these four companies, including more than $30 million from Altria alone, according to McKinsey billing records. This summer, the F.D.A., after years of criticism for not doing enough to protect the public from nicotine addiction, issued two major directives: It ordered Juul off the market over questions of safety, and proposed reducing nicotine in cigarettes to levels where consumers might no longer buy them. Although the decision affecting Juul has been stayed, the actions showed how seriously the agency now views the health risks of both products.
McKinsey declined to answer certain questions about its tobacco work. In a written response, McKinsey said: “Like many other companies and industries, our approach to working on tobacco-related issues has changed significantly over the years. We have imposed ever-stricter limitations on our work in this space until last year, when we ceased tobacco-related work entirely. We ceased all work with the vaping industry in 2020.”
Left unanswered were a host of questions. Among them: How could McKinsey, with its booming health care practice, justify advising hospitals and government agencies on how to reduce health care costs and improve medical outcomes when for years its tobacco clients were filling hospital beds with the sick and dying at an enormous cost to society?
McKinsey also did not explain why it continued to advise cigarette companies long after it was well known and widely reported that their products are harmful and addictive.
With the book “When McKinsey Comes to Town” about to be published, Mr. Sternfels sent a note to veterans of the firm.
“We will not let the fear of criticism, or the possibility that we’ll make mistakes in the future, stop us from trying to help our clients take on tough challenges and make a positive difference through our work.”
Walt Bogdanich joined The Times in 2001 as investigative editor for the Business desk. Since 2003, he has worked as an investigative reporter. He has won three Pulitzer Prizes.
Michael Forsythe is a reporter on the investigations team. He was previously a correspondent in Hong Kong, covering the intersection of money and politics in China. He has also worked at Bloomberg News and is a United States Navy veteran. @PekingMike
https://www.nytimes.com/2022/09/29/business/mckinsey-tobacco-juul-opioids.html
|
Rassegna Stampa Scientifica Ottobre 2022
|
US: MMWR: NYTS: Teenagers Keep Vaping Despite Crackdowns
“High school students resumed taking the annual National Youth Tobacco Survey in school this year and 14 percent of them reported using e-cigarettes, underscoring how an upstart industry is dodging regulators’ efforts to spare a generation from nicotine addiction… One stark finding was that one in four of the high school students who were e-cigarette users reported vaping every day. Groups opposed to e-cigarettes and tobacco products were particularly troubled by one other result that reflected the highest frequency-of-use to date: Nearly half of the high school students who were vaping said they were doing so 20 to 30 days a month.” [Christina Jewett. Teenagers Keep Vaping Despite Crackdowns on E-Cigarettes, NY Times]
Teenagers Keep Vaping Despite Crackdowns on E-Cigarettes
While use among youths has fallen since the peaks of 2018-19, resumption of in-school classes this year shows students still have access to flavored, disposable vapes.

High school students reported strongly favoring fruit- and candy-flavored vapes.Credit...Gabriela Bhaskar for The New York Times
Oct. 6, 2022
High school students resumed taking the annual National Youth Tobacco Survey in school this year and 14 percent of them reported using e-cigarettes, underscoring how an upstart industry is dodging regulators’ efforts to spare a generation from nicotine addiction.
The number shows a slight change from 11 percent last year, but researchers cautioned against drawing comparisons to 2021’s survey, which was conducted differently because it took place when many schools were closed during the pandemic. The latest results were released by the Centers for Disease Control and Prevention on Thursday.
Though the age-old force of peer pressure may still be encouraging use, the percentage of high school students who reported vaping within the last 30 days was still far lower than record-high levels reached in 2019 of nearly 28 percent.
Overall, the survey found that 2.5 million middle and high school students, or about 9 percent, used e-cigarettes in the last 30 days. That puts their overall rate of use several times higher than that of adults, which is estimated at about 3 percent.
The survey, which was conducted from January through May of this year, showed that 85 percent of adolescent e-cigarette users favored vapes in fruit, dessert and candy flavors. Some mentioned PuffBar, Vuse and Juul as their favorite brand among those on the survey’s list.
But many said their favored e-cigarette brand was not one of the 13 listed. That finding highlights how nimble the industry has been in stamping an array of brand names on vapes with flavors like strawberry ice cream and fresh vanilla that are largely made in China and shipped from warehouses to corner stores and into e-commerce.
“What that shows is that playing Whac-A-Mole with a few products is not going to solve the problem,” said Vince Willmore, a spokesman for the Campaign for Tobacco-Free Kids. “As long as any flavored products are still on the market, kids are going to shift to them. To solve the problem, you have to clear the market of all flavored products.”
Read More on Smoking, Vaping and E-Cigarettes
- The Business of Addiction: McKinsey’s ties to opioid makers are well known, but for decades the consulting giant worked with Big Tobacco and has also advised Juul.
- Youth Vaping Settlement: Juul tentatively agreed to pay $438.5 millionto settle an investigation by nearly three dozen states over marketing and sales practices that they contend set off the teen vaping crisis.
- Ban Suspension: The Food and Drug Administration ordered Juul to stop selling e-cigarettes on the U.S. market, though it later suspended the banciting “scientific issues” that warrant a review of the decision.
- Nicotine Levels: The F.D.A. is planning to require tobacco companies to slash the amount of nicotine in their cigarettes. But experts warn that drastic cuts could prove disruptive and fuel underground markets.
One stark finding was that one in four of the high school students who were e-cigarette users reported vaping every day. Groups opposed to e-cigarettes and tobacco products were particularly troubled by one other result that reflected the highest frequency-of-use to date: Nearly half of the high school students who were vaping said they were doing so 20 to 30 days a month.
“That’s a real signal of addiction and setting up young people for a lifetime of addiction which they don’t want, they didn’t choose and they don’t like,” said Robin Koval, president of the Truth Initiative, a nonprofit organization aimed at eliminating youth tobacco use.
Linda Neff, chief of the epidemiology branch in the C.D.C.’s Office on Smoking and Health, said that the sheer number of young people continuing to vape suggested the agency needed to keep working to educate teens about the effects of nicotine addiction, which “harms the parts of the brain that control learning, mood and impulse control.”
“The frequency of use is disturbing,” she said. “It’s alarming.”
The Food and Drug Administration considers e-cigarettes to be generally beneficial to the extent that they provide an alternative to adult users of traditional cigarettes, which coat the lungs in tar. The agency’s hope for health gains, though, has existed in the shadow of a youth vaping crisis that exploded in 2018-19, prompting an outcry from parents, schools, lawmakers and public health experts.
The F.D.A. began to crack down on vape makers in 2019, banning many flavors and ordering manufacturers to apply for marketing authorization to keep their products on the market — an ongoing process. That effort has been challenged by e-cigarette makers who saw a loophole in making e-cigarettes with synthetic nicotine and jumped into the market with blueberry, kiwi and candy-flavored vapes.
This spring Congress gave the F.D.A. the authority to rein in those devices. The agency said it was reviewing about one million applications to sell synthetic nicotine products. In July, the agency gained authority to remove unauthorized non-tobacco products from the market but has said it needs to move methodically as it enforces the law.
On Thursday, the agency announced that it sent new warning letters to two companies that teenagers singled out as go-to brands in the survey. The F.D.A. issued its second warning to the maker of Puff Bar vapes, this time about its flavored synthetic nicotine products that the agency said were being sold illegally.
The F.D.A. also said Thursday that it denied marketing authorization to Hyde, a company that about 5 percent of adolescents wrote in on the survey as a favored brand — suggesting the rate is higher. The company’s website shows flavors including “pink burst” and “lemon drop.”
In a statement, the agency said that Hyde must stop selling its products or “risk enforcement action.”
Juul Labs, which had been widely blamed for fueling the teenage vaping crisis, pointed to the decline in popularity of its products among youths in a statement it released on Thursday. The company is awaiting a decision from the F.D.A. on its marketing application to remain on the market.
To some, the enforcement drive by the F.D.A. appears self-defeating. Amanda Wheeler, president of the American Vapor Manufacturers, said that the flood of denials faced by U.S. vape businesses were opening the door to foreign companies that would be more difficult to regulate.
As the agency’s rejections mount, “we will continue to see black market actors take advantage of F.D.A.’s wholesale destruction of the category,” Ms. Wheeler said.
The health consequences for teenagers who develop a nicotine addiction are just beginning to be understood. Dr. Rose Marie Robertson, science and medicine officer with the American Heart Association, said scientists were seeing toxic effects from the inhaled flavoring ingredients of e-cigarettes. She said researchers were also detecting signs of use on the heart and lungs.
“It took us 40 years to show that women would develop lung cancer more readily if they smoked,” Dr. Robertson said. “The fact that we’re seeing any effects at an early stage is very worrisome.”
The persistent rate of e-cigarette use among teenagers also concerns experts who were thrilled to see youth cigarette smoking rates fall steadily for years and remain in the single digits, with about 3.3 percent of middle and high school students reporting use in 2020.
“To have decades of progress wiped away by e-cigarettes has been astonishing to us who’ve been there all along,” Dr. Robertson said.
The full results of the survey, which will include levels of other tobacco product use, is expected out later this year.
Christina Jewett covers the Food and Drug Administration. She is an award-winning investigative journalist and has a strong interest in how the work of the F.D.A. affects the people who use regulated products. @By_Cjewett
https://www.nytimes.com/2022/10/06/health/teenage-vaping-e-cigarettes.html
Big Tobacco Heralds a Healthier World While Fighting Its Arrival
The industry continues to fight efforts to restrict certain products, like spending heavily to urge California voters to overturn a law banning tobacco flavors.
The fight shaping up over government restrictions on menthol and nicotine highlights the longstanding resistance of the tobacco industry to regulations, despite corporate claims of support for a smokeless alternative. [Photo Cutline]
By Julie Creswell and Matt Richtel
Nov. 6, 2022
For decades, public health advocates chipped away at the influence of Big Tobacco with measures aimed at discouraging cigarette use. But the bitter legal and political battles were just a prelude to the unfolding climactic clash that could determine the fate of smoking and whether these companies adapt or falter.
U.S. health officials have launched the most aggressive attack by far on cigarettes: Twin government proposals would ban menthol-flavored cigarettes and would limit nicotine levels to make traditional smoking less addictive. At the same time, the government is slowly embracing vaping as an alternative by authorizing the sale of some e-cigarettes, which can provide smokers a nicotine fix without many of the carcinogens.
The measures are the source of a clash expected to play out over the coming months and years in courtrooms, legislative hallways and regulatory hearings. For public health advocates, the steps are aimed at saving millions of lives and reducing the billions of dollars spent on smoking-related illnesses like cancer and heart disease.
Big Tobacco has said it embraces the transition — sort of.
“We have an unprecedented opportunity to move beyond smoking,” Billy Gifford, chief executive of Altria, one of the world’s biggest cigarette conglomerates and the parent company of Philip Morris USA, told Wall Street analysts and investors in late October. The opening slide of his presentation offered a company vision: “To responsibly lead the transition of adult smokers to a smoke-free future.”
Major cigarette companies, like Altria and R.J. Reynolds, acknowledge that cigarettes are dangerous and addictive, and they are heralding their investments in electronic cigarettes and other less-harmful alternatives to cigarettes. But, with much less fanfare, they are taking steps to slow the very smokeless future they claim to want: The companies have submitted letters protesting the proposed menthol ban in traditional cigarettes, and they have signaled they will similarly resist any efforts to lower nicotine levels.
And Big Tobacco isn’t just duking it out at the federal level, but fighting local initiatives. For example, in California, the industry has spent heavily to stop a 2020 law from taking effect that would ban the sale of flavored-tobacco products including menthol. Putting the law in place depends on a majority of state voters supporting a Nov. 8 ballot proposition favoring the law, and the industry has spent $22 million to to try to persuade voters to reject the measure and the flavor ban.
The California Coalition for Fairness, the tobacco industry-funded group behind the campaign that succeeded in getting the referendum on the ballot, argues the flavor ban “benefits the wealthy and special interests while costing jobs and cutting funding for education and health care.”
Mr. Gifford, in his late October call with investors, said of the flavor ban: We don’t believe science supports it.”
In various statements, R.J. Reynolds, owned by British American Tobacco and the second-largest cigarette company in the United States after Altria, has said it also embraces less harm but continues to hew to a business model that critics say puts public health second to profits.
In Reynolds’s filing against the menthol ban, it wrote that, broadly, it “fully supports F.D.A.’s goal of reducing tobacco-related disease.” But, it contended, “menthol smokers would simply switch to nonmenthol cigarettes or turn to riskier options such as illicit market cigarettes.” The company declined further comment beyond its filing.
As the smoking population in the United States has fallen to 13 percent from 21 percent in 2005, far from a peak of about 45 percent of adults in 1954, and public opinion has turned against cigarettes, the legal and political might of Big Tobacco has shrunk, too. A Gallup survey conducted in July found that 74 percent of Americans favored “requiring tobacco companies to lower nicotine levels in cigarettes to make them less addictive.” About 42 percent favored banning menthol-flavored cigarettes. (Under the current proposal, menthol e-cigarettes could be sold.)
But the industry still earns billions of dollars in revenues, and it hopes to use its remaining clout to stall these monumental proposals at the regulatory level and in court — or stop them altogether.
“This spring and summer, I would say, we’ve seen the most significant period of proposed regulations by the F.D.A. ever. Full stop,” said Sarah Milov, an associate professor of history at the University of Virginia and author of “The Cigarette: A Political History.” “With this industry, it’s all about where they are making their money. We will see them fight the menthol and nicotine rules, and that will be another demonstration of their continued commitment to combustible cigarettes.”
A broad group of allies has joined the tobacco industry in the fight against the menthol ban by. There are those with financial stakes in the outcome, like the National Association of Convenience Stores, which say they would lose billions of dollars in annual sales, and the New York City Newsstand Operators Association.
The menthol ban has also drawn opposition from think tanks like the Tax Foundation, which said federal and state governments could lose a combined $6.6 billion in tax revenues the first year. The American Civil Liberties Union has also opposed the ban, saying it would disproportionately affect communities of color.
Major cigarette companies, like Altria and R.J. Reynolds, submitted letters last summer protesting the proposed menthol ban in traditional cigarettes. [Photo Cutline]
In particular, the proposed ban has divided Black leaders across the country, especially since companies heavily marketed menthol cigarettes to Black smokers, who now prefer them at a much higher rate than white smokers do. While some welcomed the proposal as a way to lower cancer and heart disease, others expressed concerns that enforcing such a ban would lead to unwarranted police interactions with Black Americans. Big Tobacco has heavily lobbied against the ban with Black political leaders and retained some to help sew doubt and fear about the ban in communities around the country.
Many opponents have challenged the F.D.A.’s legal authority to regulate tobacco products in far-reaching ways. But no matter how the companies promote their position, industry critics say that their goal is to maintain the lucrative share of the cigarette market at all costs. No wonder: Sales in the U.S. totaled $65 billion in 2021 —- one-third of it from menthol — dwarfing sales of e-cigarettes.
“It’s absolutely false that they want to have their smoking customers quit or shift to less harmful tobacco products,” said Eric Lindblom, a senior scholar at the O’Neill Institute for National and Global Health Law at Georgetown University and a former adviser to the F.D.A. “If they were serious about having smokers quit, they would stop opposing any efforts at the federal, state and local level to regulate and tax smoking tobacco products more sharply.”
Traditional cigarettes have become more expensive, though. A study published this year in JAMA found that from 2015 to 2021, the number of packs of cigarettes sold in the United States fell to 9.1 billion a year from 12.5 billion, a 27 percent drop. To compensate, tobacco companies increased prices — rising 29.5 percent a pack during that period, to $7.22 from $5.57.
Inflation plays a role, too. In the first nine months of this year, Altria reported a steep 9 percent decline in sales volumes, with executives noting that customers were changing behaviors to save money, like buying single packs of cigarettes, rather than cartons.
Company share prices have also fallen.
“Most investors knew new regulation was coming, but the threat seemed far into the future,” said Christopher Growe, an analyst at financial services firm Stifel Financial. “I think menthol has more immediacy, but nicotine regulation is a long, long way away.”
The transformation of tobacco
On some level, the battle over menthol and nicotine limits extends the government’s efforts to chip away at smoking, even as the industry resists at every turn. But this moment is also fundamentally different. For the first time, many public health officials have embraced a strategy of harm reduction, which is not just to curb the cigarette market but to accept and even advocate for an alternative with e-cigarettes.
This strategy is not one that public health officials adopted lightly: For years, many were skeptical about legalizing e-cigarettes, worrying that the devices hooked a new generation on nicotine and lured young people into the vaping crisis.
Twin government proposals involve outlawing menthol-flavored cigarettes and limiting nicotine in cigarettes. At the same time, the government is slowly embracing an alternative by legalizing the sale of some e-cigarettes. [Photo Cutline]
While public health experts debated the merits of e-cigarettes, major companies argued that, absent that alternative or other products, there were no appealing options to help smokers quit.
Mitch Zeller, who retired this year from his post as director of the F.D.A.’s Center for Tobacco Products, said that for all his experience with the companies, he wasn’t sure that they would accept a smokeless future. How they respond to the new proposals will be “a test of their sincerity,” he said.
“It’s a day of reckoning for the industry,” Mr. Zeller said, adding of the tobacco companies. “They’ve got to make a decision.”
He acknowledged that the tobacco companies were in a tough position, having widely deployed “rhetoric” supporting alternatives but, at the same time, having to answer to shareholders whose returns were still reliant on cigarette sales and profits.
“They have a fiduciary duty to their shareholders,” he said. He added, however, that regulation might force the companies to adapt, no matter how hard they resisted.
Still, tobacco giants are pushing back against any efforts to curb sales. The industry has persistently sued to stop the federal government from requiring larger, graphic warnings on packages about the deadly risk of cigarettes. And Big Tobacco companies have continued to ply a tactic they’ve used for years: poaching former F.D.A. employees, mostly recently with Philip Morris International hiring Matt Holman, who was the chief of the science office in the agency’s Center For Tobacco Products.
The tobacco industry has been joined in the struggle against the menthol ban by broad group of allies, like the National Association of Convenience Stores and the NYC Newsstand Operators Association. [Photo Cutline]
If the F.D.A. pushes through a menthol ban, the tobacco industry will “dig in” and go to court, said Marc Scheineson, a former associate commissioner at the agency who is now a partner at the law firm Alston & Bird, which represents some smaller tobacco companies. “If there are rules that are put in place with the F.D.A. sort of ignoring valid scientific objections or criticisms, it will end up in court again.”
He noted a recent win for the Cigar Association of America, which challenged the F.D.A.’s regulation of premium cigars. In that case, U.S. District Judge Amit Mehta in Washington, D.C., said the F.D.A. had acted “arbitrarily and capriciously” and ignored or overlooked evidence provided by the industry. The case is still pending.
In another blow to the F.D.A., the U.S. Court of Appeals for the 11th Circuit in late August set aside marketing denial orders for six e-cigarette companies, saying the agency there, too, had been arbitrary and capricious in its decisions.
Mr. Scheineson said he hoped a compromise could be reached. He asked: Could nicotine be reduced in a slow, laddered way while allowing menthol cigarettes to be sold?
In the interim, all e-cigarette companies have had to apply to the F.D.A. to remain on the market, now that the agency has received expanded authority to regulate vaping devices and e-cigarettes. The F.D.A. is wading through applications for 350 products, according to a letter published in Augustby Brian King, the director for the Center for Tobacco Products. In the last two years, the agency has authorized the sale of about two dozen vaping products.
And the biggest tobacco companies are vying for their piece of the budding market. Last year, the F.D.A. approved several Vuse products by Reynolds. However, the agency has not yet ruled on the sale of Vuse Alto, the company’s biggest seller to date, which accounted for 95 percent of its e-cigarette sales last year and displaced Juul as the top-selling vaping product. Vuse Alto has gained in popularity in recent years for its small, sleek design, longer battery life and the fact that it wasn’t mired in the same teenage-use controversy as Juul.
Altria’s strategy had long appeared to be pinned to its relationship with Juul Labs. In 2018, Altria paid $12.8 billion for a 35 percent stake in Juul. But even before Juul lost its initial bid in June for authorization to keep selling certain products on the U.S. market, the company’s products had been severely restricted by public pressure to pull flavored e-pods off the market out of concerns for their appeal to teenagers. The F.D.A. reversed itself this summer and is granting an additional review to Juul’s application for certain tobacco and menthol products to stay on the market.
By late September, Altria had taken a more than $12 billion cumulative loss on Juul, valuing the investment at $350 million. Altria said it ended its noncompete agreement with Juul, opening up the possibility it could acquire another e-cigarette company to compete in the space, some analysts predict. Meanwhile, reports emerged in October that Juul might seek bankruptcy protection.
Besides Juul, Altria also has stakes in companies that make nicotine pouches, a product that is placed between the cheek and jaw.
Another category of cigarette alternatives are known as “heat-not-burn tobacco sticks.” In October, Altria announced that it sold the U.S. rights to sell IQOS, a heat-not-burn tobacco stick, for $2.7 billion to Philip Morris International.
To fill the void, Altria promptly announced a new joint venture with Japan Tobacco to develop a heat-not-burn stick called Ploom for the U.S. market.
On Altria’s call with investors in late October, Mr. Growe, the Wall Street analyst from Stifel, asked the company’s chief executive when a new Ploom product might be available. “Do you have a reasonable time frame for launching a product in the U.S.” he asked, and then added a few sentences later: “Or am I getting ahead of myself here?”
“I think you’re getting ahead of yourself a little bit,” said Mr. Gifford, Altria’s chief executive.
“Maybe underlying your question is: ‘why are you taking so long,” Mr. Gifford continued. “And I think it goes back to, look, we want to be disciplined.”
Mr. Gifford said that Altria absolutely wants to create an alternative to the cigarette, but not in a hasty fashion. “We need to go about it in a thoughtful manner.”
Julie Creswell is a New York-based reporter. She has covered banks, private equity, retail and health care. She previously worked for Fortune Magazine and also wrote about debt, monetary policy and mutual funds at Dow Jones. @julie_creswell
Matt Richtel is a best-selling author and Pulitzer Prize-winning reporter based in San Francisco. He joined The Times in 2000, and his work has focused on science, technology, business and narrative-driven storytelling around these issues. @mrichtel
https://www.nytimes.com/2022/11/06/health/tobacco-fda-menthol-ban-nicotine.html
|
Rassegna Stampa Scientifica Novembre 2022
|
Big Tobacco Heralds a Healthier World While Fighting Its Arrival
The industry continues to fight efforts to restrict certain products, like spending heavily to urge California voters to overturn a law banning tobacco flavors.
The fight shaping up over government restrictions on menthol and nicotine highlights the longstanding resistance of the tobacco industry to regulations, despite corporate claims of support for a smokeless alternative. [Photo Cutline]
By Julie Creswell and Matt Richtel
Nov. 6, 2022
For decades, public health advocates chipped away at the influence of Big Tobacco with measures aimed at discouraging cigarette use. But the bitter legal and political battles were just a prelude to the unfolding climactic clash that could determine the fate of smoking and whether these companies adapt or falter.
U.S. health officials have launched the most aggressive attack by far on cigarettes: Twin government proposals would ban menthol-flavored cigarettes and would limit nicotine levels to make traditional smoking less addictive. At the same time, the government is slowly embracing vaping as an alternative by authorizing the sale of some e-cigarettes, which can provide smokers a nicotine fix without many of the carcinogens.
The measures are the source of a clash expected to play out over the coming months and years in courtrooms, legislative hallways and regulatory hearings. For public health advocates, the steps are aimed at saving millions of lives and reducing the billions of dollars spent on smoking-related illnesses like cancer and heart disease.
Big Tobacco has said it embraces the transition — sort of.
“We have an unprecedented opportunity to move beyond smoking,” Billy Gifford, chief executive of Altria, one of the world’s biggest cigarette conglomerates and the parent company of Philip Morris USA, told Wall Street analysts and investors in late October. The opening slide of his presentation offered a company vision: “To responsibly lead the transition of adult smokers to a smoke-free future.”
Major cigarette companies, like Altria and R.J. Reynolds, acknowledge that cigarettes are dangerous and addictive, and they are heralding their investments in electronic cigarettes and other less-harmful alternatives to cigarettes. But, with much less fanfare, they are taking steps to slow the very smokeless future they claim to want: The companies have submitted letters protesting the proposed menthol ban in traditional cigarettes, and they have signaled they will similarly resist any efforts to lower nicotine levels.
And Big Tobacco isn’t just duking it out at the federal level, but fighting local initiatives. For example, in California, the industry has spent heavily to stop a 2020 law from taking effect that would ban the sale of flavored-tobacco products including menthol. Putting the law in place depends on a majority of state voters supporting a Nov. 8 ballot proposition favoring the law, and the industry has spent $22 million to to try to persuade voters to reject the measure and the flavor ban.
The California Coalition for Fairness, the tobacco industry-funded group behind the campaign that succeeded in getting the referendum on the ballot, argues the flavor ban “benefits the wealthy and special interests while costing jobs and cutting funding for education and health care.”
Mr. Gifford, in his late October call with investors, said of the flavor ban: We don’t believe science supports it.”
In various statements, R.J. Reynolds, owned by British American Tobacco and the second-largest cigarette company in the United States after Altria, has said it also embraces less harm but continues to hew to a business model that critics say puts public health second to profits.
In Reynolds’s filing against the menthol ban, it wrote that, broadly, it “fully supports F.D.A.’s goal of reducing tobacco-related disease.” But, it contended, “menthol smokers would simply switch to nonmenthol cigarettes or turn to riskier options such as illicit market cigarettes.” The company declined further comment beyond its filing.
As the smoking population in the United States has fallen to 13 percent from 21 percent in 2005, far from a peak of about 45 percent of adults in 1954, and public opinion has turned against cigarettes, the legal and political might of Big Tobacco has shrunk, too. A Gallup survey conducted in July found that 74 percent of Americans favored “requiring tobacco companies to lower nicotine levels in cigarettes to make them less addictive.” About 42 percent favored banning menthol-flavored cigarettes. (Under the current proposal, menthol e-cigarettes could be sold.)
But the industry still earns billions of dollars in revenues, and it hopes to use its remaining clout to stall these monumental proposals at the regulatory level and in court — or stop them altogether.
“This spring and summer, I would say, we’ve seen the most significant period of proposed regulations by the F.D.A. ever. Full stop,” said Sarah Milov, an associate professor of history at the University of Virginia and author of “The Cigarette: A Political History.” “With this industry, it’s all about where they are making their money. We will see them fight the menthol and nicotine rules, and that will be another demonstration of their continued commitment to combustible cigarettes.”
A broad group of allies has joined the tobacco industry in the fight against the menthol ban by. There are those with financial stakes in the outcome, like the National Association of Convenience Stores, which say they would lose billions of dollars in annual sales, and the New York City Newsstand Operators Association.
The menthol ban has also drawn opposition from think tanks like the Tax Foundation, which said federal and state governments could lose a combined $6.6 billion in tax revenues the first year. The American Civil Liberties Union has also opposed the ban, saying it would disproportionately affect communities of color.
Major cigarette companies, like Altria and R.J. Reynolds, submitted letters last summer protesting the proposed menthol ban in traditional cigarettes. [Photo Cutline]
In particular, the proposed ban has divided Black leaders across the country, especially since companies heavily marketed menthol cigarettes to Black smokers, who now prefer them at a much higher rate than white smokers do. While some welcomed the proposal as a way to lower cancer and heart disease, others expressed concerns that enforcing such a ban would lead to unwarranted police interactions with Black Americans. Big Tobacco has heavily lobbied against the ban with Black political leaders and retained some to help sew doubt and fear about the ban in communities around the country.
Many opponents have challenged the F.D.A.’s legal authority to regulate tobacco products in far-reaching ways. But no matter how the companies promote their position, industry critics say that their goal is to maintain the lucrative share of the cigarette market at all costs. No wonder: Sales in the U.S. totaled $65 billion in 2021 —- one-third of it from menthol — dwarfing sales of e-cigarettes.
“It’s absolutely false that they want to have their smoking customers quit or shift to less harmful tobacco products,” said Eric Lindblom, a senior scholar at the O’Neill Institute for National and Global Health Law at Georgetown University and a former adviser to the F.D.A. “If they were serious about having smokers quit, they would stop opposing any efforts at the federal, state and local level to regulate and tax smoking tobacco products more sharply.”
Traditional cigarettes have become more expensive, though. A study published this year in JAMA found that from 2015 to 2021, the number of packs of cigarettes sold in the United States fell to 9.1 billion a year from 12.5 billion, a 27 percent drop. To compensate, tobacco companies increased prices — rising 29.5 percent a pack during that period, to $7.22 from $5.57.
Inflation plays a role, too. In the first nine months of this year, Altria reported a steep 9 percent decline in sales volumes, with executives noting that customers were changing behaviors to save money, like buying single packs of cigarettes, rather than cartons.
Company share prices have also fallen.
“Most investors knew new regulation was coming, but the threat seemed far into the future,” said Christopher Growe, an analyst at financial services firm Stifel Financial. “I think menthol has more immediacy, but nicotine regulation is a long, long way away.”
The transformation of tobacco
On some level, the battle over menthol and nicotine limits extends the government’s efforts to chip away at smoking, even as the industry resists at every turn. But this moment is also fundamentally different. For the first time, many public health officials have embraced a strategy of harm reduction, which is not just to curb the cigarette market but to accept and even advocate for an alternative with e-cigarettes.
This strategy is not one that public health officials adopted lightly: For years, many were skeptical about legalizing e-cigarettes, worrying that the devices hooked a new generation on nicotine and lured young people into the vaping crisis.
Twin government proposals involve outlawing menthol-flavored cigarettes and limiting nicotine in cigarettes. At the same time, the government is slowly embracing an alternative by legalizing the sale of some e-cigarettes. [Photo Cutline]
While public health experts debated the merits of e-cigarettes, major companies argued that, absent that alternative or other products, there were no appealing options to help smokers quit.
Mitch Zeller, who retired this year from his post as director of the F.D.A.’s Center for Tobacco Products, said that for all his experience with the companies, he wasn’t sure that they would accept a smokeless future. How they respond to the new proposals will be “a test of their sincerity,” he said.
“It’s a day of reckoning for the industry,” Mr. Zeller said, adding of the tobacco companies. “They’ve got to make a decision.”
He acknowledged that the tobacco companies were in a tough position, having widely deployed “rhetoric” supporting alternatives but, at the same time, having to answer to shareholders whose returns were still reliant on cigarette sales and profits.
“They have a fiduciary duty to their shareholders,” he said. He added, however, that regulation might force the companies to adapt, no matter how hard they resisted.
Still, tobacco giants are pushing back against any efforts to curb sales. The industry has persistently sued to stop the federal government from requiring larger, graphic warnings on packages about the deadly risk of cigarettes. And Big Tobacco companies have continued to ply a tactic they’ve used for years: poaching former F.D.A. employees, mostly recently with Philip Morris International hiring Matt Holman, who was the chief of the science office in the agency’s Center For Tobacco Products.
The tobacco industry has been joined in the struggle against the menthol ban by broad group of allies, like the National Association of Convenience Stores and the NYC Newsstand Operators Association. [Photo Cutline]
If the F.D.A. pushes through a menthol ban, the tobacco industry will “dig in” and go to court, said Marc Scheineson, a former associate commissioner at the agency who is now a partner at the law firm Alston & Bird, which represents some smaller tobacco companies. “If there are rules that are put in place with the F.D.A. sort of ignoring valid scientific objections or criticisms, it will end up in court again.”
He noted a recent win for the Cigar Association of America, which challenged the F.D.A.’s regulation of premium cigars. In that case, U.S. District Judge Amit Mehta in Washington, D.C., said the F.D.A. had acted “arbitrarily and capriciously” and ignored or overlooked evidence provided by the industry. The case is still pending.
In another blow to the F.D.A., the U.S. Court of Appeals for the 11th Circuit in late August set aside marketing denial orders for six e-cigarette companies, saying the agency there, too, had been arbitrary and capricious in its decisions.
Mr. Scheineson said he hoped a compromise could be reached. He asked: Could nicotine be reduced in a slow, laddered way while allowing menthol cigarettes to be sold?
In the interim, all e-cigarette companies have had to apply to the F.D.A. to remain on the market, now that the agency has received expanded authority to regulate vaping devices and e-cigarettes. The F.D.A. is wading through applications for 350 products, according to a letter published in Augustby Brian King, the director for the Center for Tobacco Products. In the last two years, the agency has authorized the sale of about two dozen vaping products.
And the biggest tobacco companies are vying for their piece of the budding market. Last year, the F.D.A. approved several Vuse products by Reynolds. However, the agency has not yet ruled on the sale of Vuse Alto, the company’s biggest seller to date, which accounted for 95 percent of its e-cigarette sales last year and displaced Juul as the top-selling vaping product. Vuse Alto has gained in popularity in recent years for its small, sleek design, longer battery life and the fact that it wasn’t mired in the same teenage-use controversy as Juul.
Altria’s strategy had long appeared to be pinned to its relationship with Juul Labs. In 2018, Altria paid $12.8 billion for a 35 percent stake in Juul. But even before Juul lost its initial bid in June for authorization to keep selling certain products on the U.S. market, the company’s products had been severely restricted by public pressure to pull flavored e-pods off the market out of concerns for their appeal to teenagers. The F.D.A. reversed itself this summer and is granting an additional review to Juul’s application for certain tobacco and menthol products to stay on the market.
By late September, Altria had taken a more than $12 billion cumulative loss on Juul, valuing the investment at $350 million. Altria said it ended its noncompete agreement with Juul, opening up the possibility it could acquire another e-cigarette company to compete in the space, some analysts predict. Meanwhile, reports emerged in October that Juul might seek bankruptcy protection.
Besides Juul, Altria also has stakes in companies that make nicotine pouches, a product that is placed between the cheek and jaw.
Another category of cigarette alternatives are known as “heat-not-burn tobacco sticks.” In October, Altria announced that it sold the U.S. rights to sell IQOS, a heat-not-burn tobacco stick, for $2.7 billion to Philip Morris International.
To fill the void, Altria promptly announced a new joint venture with Japan Tobacco to develop a heat-not-burn stick called Ploom for the U.S. market.
On Altria’s call with investors in late October, Mr. Growe, the Wall Street analyst from Stifel, asked the company’s chief executive when a new Ploom product might be available. “Do you have a reasonable time frame for launching a product in the U.S.” he asked, and then added a few sentences later: “Or am I getting ahead of myself here?”
“I think you’re getting ahead of yourself a little bit,” said Mr. Gifford, Altria’s chief executive.
“Maybe underlying your question is: ‘why are you taking so long,” Mr. Gifford continued. “And I think it goes back to, look, we want to be disciplined.”
Mr. Gifford said that Altria absolutely wants to create an alternative to the cigarette, but not in a hasty fashion. “We need to go about it in a thoughtful manner.”
Julie Creswell is a New York-based reporter. She has covered banks, private equity, retail and health care. She previously worked for Fortune Magazine and also wrote about debt, monetary policy and mutual funds at Dow Jones. @julie_creswell
Matt Richtel is a best-selling author and Pulitzer Prize-winning reporter based in San Francisco. He joined The Times in 2000, and his work has focused on science, technology, business and narrative-driven storytelling around these issues. @mrichtel
https://www.nytimes.com/2022/11/06/health/tobacco-fda-menthol-ban-nicotine.html
|
Rassegna Stampa Scientifica Dicembre 2022
|
“New Zealand has introduced a steadily rising smoking age to stop those aged 14 and under from ever being able to legally buy cigarettes in world-first legislation to outlaw smoking for the next generation. Associate health minister Ayesha Verrall said at the law’s passing on Tuesday: “Thousands of people will live longer, healthier lives and the health system will be $5bn better off from not needing to treat the illnesses caused by smoking, such as numerous types of cancer, heart attacks, strokes, amputations.” New Zealand is believed to be the first country in the world to implement the annually rising smoking age, ensuring tobacco cannot be sold to anyone born on or after 1 January 2009. It will be accompanied by a slew of other measures to make smoking less affordable and accessible, including dramatically reducing the legal amount of nicotine in tobacco products and forcing them to be sold only through specialty tobacco stores, rather than corner stores and supermarkets.” [Tess McClure. New Zealand passes world-first tobacco law to ban smoking for next generation, The Guardian]
“The Supreme Court [this past] Monday refused to block a California law banning flavored tobacco, clearing the way for the ban to take effect next week… R.J. Reynolds, the maker of Newport menthol cigarettes, had asked the justices to intervene before next Wednesday, when the law is set to go into effect. The company, joined by several smaller ones, argued that a federal law, the Tobacco Control Act of 2009, allows states to regulate tobacco products but prohibits banning them… Last week, the Justice Department announced an agreement for 200,000 retailers to display eye-catching signs in their stores about the dangers of cigarette smoking. The order goes into effect in July and gives retailers three months to post the signs. The agreement settles the terms of a 1999 racketeering lawsuit filed by the U.S. government against tobacco companies, including Reynolds.” [Adam Liptak. Supreme Court Refuses to Block California’s Ban on Flavored Tobacco, NY Times. See also: U.S. Supreme Court lets California ban flavored tobacco products, Reuters]
“Juul Labs, the e-cigarette manufacturer, announced [last] Tuesday that it has reached settlements covering more than 5,000 cases with nearly 10,000 plaintiffs. The sweeping resolutions, which litigators say will address youth e-cigarette usage, come after more than three years of legal battles. The settlements include compensation for those suffering from nicotine addiction and other health problems as well as reimbursement for those who purchased Juul products... Juul did not disclose the settlement amount… But Robert Jackler, a Stanford medical school professor who researches the impact of tobacco advertising and served as an expert witness in the proceedings, was less optimistic. “Time and again, tobacco companies absorb sizable legal settlements as a cost of doing business, only [to] reemerge as highly profitable purveyors [of] nicotine products, which they market to youth.”” [Kelsey Ables. E-cigarette firm Juul settles 5,000 lawsuits amid teen vaping concerns, Washington Post. See also: Juul settles more than 5,000 lawsuits over its vaping products, NPR]
“Using e-cigarettes to quit was associated with significantly lower odds of having stopped smoking cigarettes (odds ratio, 0.62; 95% confidence interval, 0.45–0.85), controlling for nicotine dependence and demographics… Ever-smoking youth who used e-cigarettes “to try to quit using other tobacco products, such as cigarettes” had lower odds of having stopped smoking cigarettes than those who did not use e-cigarettes as to try to quit. Physicians, regulators, and educators should discourage youth from attempting to use e-cigarettes as a way to stop smoking cigarettes.”
e-Cigarettes Used by Adolescents to Try to Quit Smoking Are Associated With Less Quitting: A Cross-Sectional Analysis of the National Youth Tobacco Survey
Journal of Adolescent Health
Available online 5 December 2022
In Press, Corrected Proof
Stanton A. Glantz.
https://www.sciencedirect.com/science/article/abs/pii/S1054139X22007066
“The growing use of e-cigarettes has been condemned as a significant health crisis by some and welcomed as an unprecedented opportunity to eliminate combustible tobacco by others. Seeking to better understand the contestation and range of perspectives on this issue, this article employs an interpretivist approach to identify how experts communicate their perspectives on these issues… Throughout, experts struggled and disagreed with precisely where and how to define “harm reduction." Overall, this study significantly expands on past literature by delving more deeply into the broader ideological contexts in which these policy disagreements occur, and the argumentative strategies employed within them.”
Understanding experts’ conflicting perspectives on tobacco harm reduction and e-cigarettes: An interpretive policy analysis
SSM - Qualitative Research in Health, 2, 100197.
Available online 24 November 2022, Version of Record 2 December 2022.
Daniel Eisenkraft Klein, Benjamin Hawkins, Robert Schwartz
https://www.sciencedirect.com/science/article/pii/S2667321522001597
Note: Open Access.
[Kenyan farmer] Siprone Chacha “was tempted into tobacco cultivation by the prospect of increasing her income. However, she found that by the time she had cut down trees, cleared land, planted the crop, paid for the pesticides and the fertilizer, and then carefully nurtured the crop to maturity it was hard to make a profit. On top of everything else, the buyer with whom she had a contract did not accept all of her leaves. “If the leaf was not perfect, he would not take it which meant that I had to dump the unsold crop on my land which was poisoned by the nicotine.”
Cultivating tobacco-free farms
Bull World Health Organ. 2022 Dec 1; 100(12): 754–755.
Gary Humphreys
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9706357/
Note: Open Access.
Pagina 4 di 8





















